Hamid Irannejad
Mazandaran University of Medical Sciences, Iran
Title: Neuroprotective activity of novel 5,6-Diaryl-1,2,4-triazine derivatives with ethyl acetate moiety against H2O2 and Aβ-induced neurotoxicity
Biography
Biography: Hamid Irannejad
Abstract
Alzheimer’s disease (AD) is a neuropathologic disorder characterized by intracellular neurofibrillary tangles and amyloid aggregates in the CNS. In recent years numerous approaches have been used to combat AD like small molecule inhibitors of Aβ aggregation, anti-inflammatory agents, cholinesterase, a- and β-secretase.
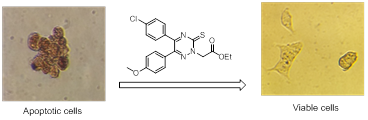
Herein, we report synthesis of some 5,6-diaryl-1,2,4-triazines 3a-f and 8a-e as potential agents for treatment of AD. We evaluated them against both H2O2 and β-amyloid induced toxicity in PC-12 and SH-SY5Y cells and the extent of cell viability and apoptosis were assessed during 24 and 48 h of treatment.
All compounds showed significant neuroprotective activity with EC50 values ranging from 14-30 µM. Most compounds could increase cell viability compared to amyloid treated group.
Surprisingly, 3-thioxo-1,2,4-triazin-2(3H)-yl)acetate derivative 8e was the most potent compound in both tests with EC50 of 14 µM in H2O2 induced apoptosis and could increase 40% of cell viability revealed by cytometric analysis with Annexin V/PI staining. It was also shown that 8e has more neuroprotective activity than Quercetin in beta-amyloid induced toxicity. Moreover, compound 8e attenuated late-apoptosis from 42% to 6% (P<0.005) and 7% to 1% at 24 and 48 hour respectively compared to amyloid treated cells. Similarly, apoptosis was reduced from 12% to 4% at 24 hours. LDH release was not changed at any time points, pointing anti-apoptotic effect of compound 8e. Morphologic evaluation of cells by DAPI staining and TUNEL assay showed the effectiveness of this compound to improve neurite outgrowth and to prevent apoptosis and DNA fragmentation in neuronal cells.