
Giuseppe Scalabrino
University of Milan, Italy
Title: The molecular basis of the neurotrophic action of vitamin B12 (cobalamin)
Biography
Biography: Giuseppe Scalabrino
Abstract
Our experimental and clinical studies have highlighted the non-coenzyme functions of cobalamin (Cbl, vitamin B12). Cytokine and growth factor (GF) imbalance in the central nervous system (CNS) of Cbl-deficient (Cbl-D) rats is a key point in the pathogenesis of Cbl-D neuropathy. The increased molecules are tumor necrosis factor(TNF)-a, nerve GF, and the soluble (s) CD40:sCD40Ligand dyad; the decreased molecules are epidermal GF (EGF) and interleukin-6. The in vivo administration of the lacking myelinotrophic molecules or agents antagonizing the excess myelintoxic agent is as effective as Cbl in repairing or preventing Cbl-deficiency-induced CNS myelin lesions. Cbl deficiency morphologically affects also glial cells, which normally synthesize and release various cytokines and GFs. Therefore, Cbl deficiency triggers the rearrangements of glia gene expression, eventually leading to a changed pattern of cytokine and GF production. Such an opposite imbalance in TNF-a and EGF similar to that observed in CNS of Cbl-D rats has been found in the sera of adult patients with pernicious anemia (but not in patients with iron-deficient anemia), and it was rectified by Cbl therapy. This imbalance has been found also in the cerebrospinal fluid (CSF) of adult patients with Cbl-D neuropathy. Given that TNF-a and EGF regulate the expression of normal prions (PrPcs) and PrPcs play a crucial role in myelin maintenance, we investigated whether CNS PrPc levels are indirectly regulated by Cbl. PrPc levels had increased by the time Cbl-D-induced myelin lesions appeared. This increase was mediated by excess TNF-a and prevented by EGF. Cbl deficiency greatly reduced CNS PrPc-mRNA levels, which were subsequently increased by Cbl and EGF. Similar increases in PrPc levels also occur in the serum and CSF of adult Cbl-D patients, and the serum increase has been corrected by Cbl therapy. Therefore, Cbl may regulate the PrPc levels in the serum and CSF in humans.
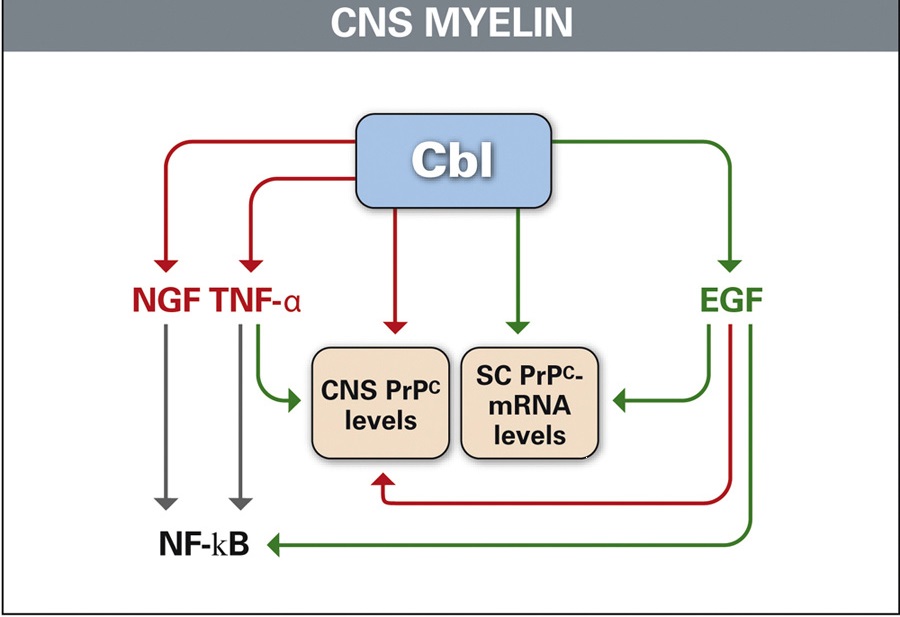
Figure 1: Cbl as a fulcrum between physiologically myelinotrophic (green) and potential myelinodamaging agents (red) in rat CNS. This is highlighted by the Cbl-mediated stimulation of EGF, the Cbl- and EGF-mediated stimulation of PrPc synthesis (green arrows), and the reduction of TNF-a and NGF levels (red arrows), eventually leading to a low NF-kB level



