Mariya Hristova
University College London, UK
Title: Inhibition of Signal Transducer and Activator of Transcription 3 (STAT3) reduces neonatal hypoxic-ischemic brain damage
Biography
Biography: Mariya Hristova
Abstract
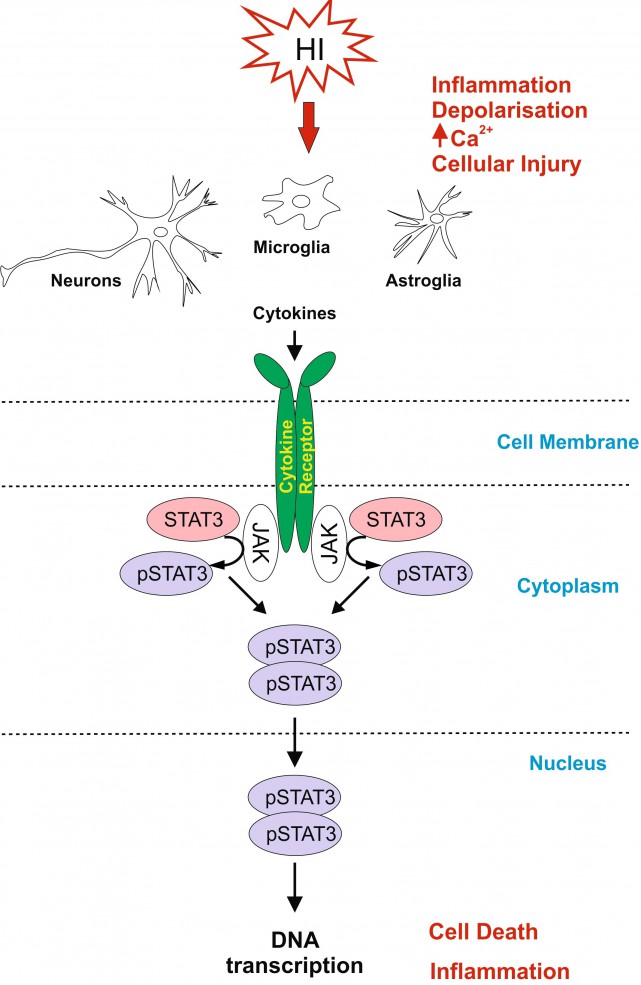
Neonatal hypoxic-ischaemic encephalopathy (HIE) is a leading cause of neonatal mortality and morbidity, affecting 1-3 per 1000 live-births in developed countries with rates about 5-10 times higher in low-income setting. About 40% of the affected children die in the neonatal period and further 30% develop life-long disabilities such as cerebral palsy, epilepsy and mental retardation. Therapeutic hypothermia is the only clinically approved care for moderate to severe neonatal hypoxic-ischaemic (HI) brain injury, however it reduces death and disability only by 11% with about 40% of the treated infants still developing neurological incapacities. For hypothermia to be effective, 7-8 infants need to be treated for one to benefit from the treatment. Therefore it is necessary to develop simple, safe and effective supplementary therapies to add to the current therapeutic strategy in infants with HIE.
Signal Transducer and Activator of Transcription 3 (STAT3) is strongly up-regulated by HI in the immature brain. To investigate the association of STAT3 up-regulation with HI-brain damage, and whether phosphorylated STAT3 originating from different cell types has a different role in promoting HI-damage, we subjected postnatal day seven mice to unilateral carotid artery ligation followed by 60min hypoxia. Neuron-specific STAT3-deletion reduced microglial and astroglial activation, cell death and tissue loss in all brain regions. Astroglial STAT3-deletion also reduced microglial activation, cell death and tissue loss, although not as much as neuronal deletion. Systemic STAT3-inhibition with JAK2-inhibitor WP1066 only moderately reduced microglial and astroglial activation, but in a pattern similar to the one observed with the cell-specific deletions. Our results suggest that STAT3 is an important factor in neonatal HI-brain damage and its removal in neurons or astrocytes, and, to some extent, systemic inhibition reduces inflammation and tissue loss. Overall, the protective effects of STAT3 inhibition make it a potential target for a therapeutic strategy in neonatal HI.