Day 1 :
Keynote Forum
Hari Shanker Sharma
University Hospital of Uppsala University, Sweden
Keynote: Blood-brain barrier in Alzheimer’s disease induced brain pathology
Time : 09:30-09:55 AM

Biography:
Hari Shanker Sharma is the Director of International Experimental Central Nervous system (CNS) Injury & Repair (IECNSIR) at University Hospital, Uppsala University, Sweden. He is a qualified neuroanatomist and experimental neurpathologist trained in Germany, Switzerland, Hungary, Sweden and USA. His main research interest is currently focused on neurotoxicity of nanoparticle and nanowired drug delivery of agents for enhanced neuroprotection in a variety of CNS insults or neurodegenerative diseases in relation to the blood-brain barrier (BBB) function. He has authored more than 250 original research papers and edited several book volumes or Progress in Brain Research series.
Abstract:
Military personnel are highly vulnerable to Alzheimer’s disease (AD). This is because of the fact that severe stress of trauma, sleep deprivation or combat stress causes increased deposition of amyloid beta peptide in the cerebrospinal fluid (CSF) and in brain parenchyma. Continued stress in military is one of the main causes of development of hypertension and mental abnormalities. Thus, exploration of novel therapeutic strategies are needed to reduce brain damage in military following stress and thwarting development of AD like pathology.
Previous studies show that stressful situations alone induce breakdown of the blood-brain barrier (BBB) and neuronal damages that is long lasting. Thus, a possibility arises that breakdown of the BBB in stress could play critical roles in development of AD. In present investigation we explored whether AD induced brain pathology caused by amyloid beta peptide (AbP) infusion is exacerbated in rats subjected to repeated immobilization that induces mild hypertension and opens the BBB to large molecules e.g., proetins.
AbP (1-40) was administered intraventricularly (i.c.v.) in the left lateral ventricle (250 ng/10 µl) of rats (250-300 g body weight) once daily for 4 weeks in naïve animals as well as in rats that were subjected to repeated immobilization stress 2 h daily for 1 week. Control rats received identical dose of saline instead of AbP. BBB breakdown, edema formation, neuronal, glial injuries and AbP deposits in the brain was examined in a blinded fashion.
Repeated 2 h stress for 1 week induced marked BBB breakdown to Evans blue albumin and radioiodine tracers in the cerebral cortex, hippocampus, thalamus, hypothalamus, caudate nucleus, cerebellum and brainstem from the naive rats. Infusion of AbP in these stressed rats further enhanced the BBB breakdown to protein tracers by several-folds and aggravation of neuronal damages, astrocytic activation and brain swelling. The number of AbP positive cells increased by 3- to 6- in stressed group as compared to naive rats. Co administration of TiO2 or PLGA nanoparticles (NPs) loaded cerebrolysin (2.5 ml/kg, i.v. /day from 2nd week of AbP infusion for 2 weeks) induced profound neuroprotection in stressed rats. It appears that TiO2-nanwored delivery has superior neuroprotective effects in this AD model as compared to PLGA-delivery of identical doses of cerebrolysin. Taken together our observations are the first to demonstrate that repeated stress exacerbates AD brain pathology and nanodelivery of cerebrolysin has superior neuroprotective effects.
Taken together, our results demonstrate that breakdown of the BBB is key to AD induced brain pathology and restoration of BBB function by cerebrolysin induces neuroprotection in AD.
Keynote Forum
Stephen D Skaper
University of Padua, Italy
Keynote: Co-ultramicronized palmitoylethanolamide/luteolin facilitates oligodendrocyte precursor cell development and improves outcome in experimental autoimmune encephalomyelitis
Time : 10:45- 11:10

Biography:
Stephen D. Skaper received a PhD in biochemistry from the University of South Dakota and Laurea in chemistry from the University of Padua, Italy. He is currently Adjunct Professor in the Department of Pharmaceutical and Pharmacological Sciences (section on Pharmacology and Anesthesiology) at the University of Padua. From 1998-2008 he was a Senior Group Leader for Neurodegeneration Research, Neurology & GI Centre of Excellence for Drug Discovery, GlaxoSmithKline Research and Development Limited, United Kingdom. Prior to that he held academic research positions in the Department of Biology at the University of California, San Diego. Dr. Skaper has authored/co-authored over 300 research papers, book chapters and monographs, as well as having guest-edited six journal thematic issues and a volume of Methods in Molecular Biology on neurotrophic factors. He is Editor-in-Chief of CNS & Neurological Disorders Drug Targets, the Associate Editor of the American Journal of Neuroprotection and Neuroregeneration, and a Councillor of the International Association of Neurorestoratology. His research interests focus on the role of immune cells and their interactions in neuroinflammation, in particular with regards to neuropathic pain and autoimmune demyelinating diseases like multiple sclerosis, and the development of therapeutic strategies based on natural molecules. He is a member of Sigma ïƒI, Phi Lambda Upsilon, Alpha Chi Sigma, the Society for Neuroscience and the International Society of Cerebral Blood Flow and Metabolism.
Abstract:
Oligodendrocytes, the myelin-producing cells of the CNS have limited ability to repair damage either to themselves or to other nerve cells. Such is the case in multiple sclerosis (MS), a chronic CNS neuroinflammatory demyelinating disorder. MS lesions are characterized by the presence of a compromised pool of undifferentiated oligodendrocyte precursor cells (OPCs) which fail to mature into myelin-producing oligodendrocytes. An attractive strategy may thus be to replace lost oligodendrocytes and/or promote their maturation or proliferation. N-palmitoylethanolamine (PEA), an endogenous fatty acid amide signaling molecule possesses analgesic, anti-inflammatory, and neuroprotective actions. Recent studies show a co-ultramicronized composite of PEA and the flavonoid luteolin (co-ultraPEALut, 10:1 by mass) to be more efficacious that PEA alone in improving outcome in CNS injury models. Here, we examined the effects of co-ultraPEALut on the survival and development of OPCs isolated from newborn rat cortical mixed glial cell cultures. OPCs were maintained under conditions which favored either proliferation (basic fibroblast factor and platelet-derived growth factor (PDGF)-AA-supplemented serum-free medium (‘SFM’)) or differentiation (Sato medium containing T3 and T4). OPCs cultured in SFM displayed high expression of PDGF receptor alpha gene and the established proliferation marker Ki67 in the presence of 10 mM co-ultraPEALut and down-regulation of Apoe, whose deletion reportedly leads to a later time of peak symptoms/disease severity and less severe demyelination/axonal damage in myelin oligodendrocyte glycoprotein (MOG35-55)-induced experimental autoimmune encephalomyelitis (EAE) in female C57BL/6 mice. In Sato medium OPCs showed rapid decreases in PDGF receptor alpha and Ki67 expression but a time-dependent rise in myelin basic protein (MBP) expression. In the latter conditions co-ultraPEALut (10 mM) enhanced OPC morphological complexity, protein content, and gene expression for MBP, proteolipid protein and 2',3'-cyclic nucleotide 3'-phosphodiesterase, as well as genes coding for enzymes involved in cholesterol and fatty acid synthesis – all important components of myelin. Co-ultraPEALut also increased OPC content of MBP. Moreover, co-ultraPEALut dose-dependentlyimproved the clinical score in this EAE mouse model, which is often used as a chronic first-pass model of MS. Hence, strategies intended to promote endogenous remyelination in MS should focus on both enhancing the long-term survival of OPCs and on stimulating these cells to differentiate into remyelinating oligodendrocytes. Within this context, co-ultraPEALut may represent a novel pharmacological approach.
Keynote Forum
Harish C Pant
National Institutes of Health, USA
Keynote: Translational approach to Neurodegenerative diseases: A small peptide derived from neuronal cell cycle kinase (Cdk5) prevents neurodegeneration
Time : 10:20-11:05

Biography:
Dr. Pant received his M.A. and Ph.D. degrees in Physics from Agra University, Agra, India. His postdoctoral studies were conducted on the mechanisms of electron and ion transport in model membrane systems at the Department of Biophysics at Michigan State University. He joined the Laboratory of Neurobiology in the NIMH as a senior staff fellow in 1974 with Dr. Ichiji Tasaki where he studied the function of the axonal cytoskeleton in the squid giant axon. In 1979 he moved to the NIAAA extending his studies on the neuronal cytoskeleton and the effects of alcohol on its regulation. Dr. Pant moved to the NINDS, Laboratory of Neurochemistry in 1987 where he is presently chief of the section on Cytoskeleton Regulation. His laboratory is studying the mechanisms of topographic regulation of neuronal cytoskeleton proteins by post-translational modification, including the role of kinase cascades in normal brain and during neurodegeneration.
Abstract:
During our studies on the compartment specific phosphorylation of cytoskeletal proteins in the neurons, we discovered a novel kinase, Cdk5, a Cell Cycle dependent like kinase in the brain. Though it binds with cyclins, however, its activity is primarily restricted to neurons due to its binding and regulation by neuron specific molecules p35 and p39 (35 KDa and 39 KDa molecular weight respectively). Cdk5, by virtue of its tightly regulated, multifunctional role in neuronal development, migration, synaptogenesis, synaptic activity, memory / learning and survival. It targets a large number of different types of neuronal proteins and has emerged as a major player in nervous system function in health and disease. However, due to neuronal insults and stress (e.g., A-beta, glutamate, oxidative, mutational, neuroinflammation and intra / extra cellular stresses, Cdk5 is hyperactivated and deregulated induces a number of neurodegenerative disorders. Although our studies continue to unravel the role of Cdk5 in neurogenesis and synaptic function but our most exciting recent results have been related to its role in neurodegeneration and our success in developing compounds that protect neurons from deregulated Cdk5 pathology, neuro-inflammation, and apoptosis in vitro and in AD and other neurodegenerative disease (ALS, PD) model mice. Hence, our current and future work include a major emphasis on the efficacy of our newly modified peptide TFP5 (carrying a fluorescent marker at the N-terminal end and a TAT PTD sequence at the C-terminal (to facilitate penetration into tissues) and pass blood brain barrier, as a therapeutic candidate for AD, ALS and PD using model mice. Currently, most therapeutic approaches targeting the deregulated Cdk5/p25 complex and other kinases in neurodegenerative disorders have focused primarily on drugs like roscovitine that inhibit kinase activity by interfering with the ATP binding domain of the kinase. Most of these drugs, however, lack sufficient specificity, since all kinases including cell cycle Cdks, are vulnerable at the ATP binding site targeted by roscovitine. We identified a 24 residue truncated modified peptide (TFP5), derived from the p35 activator, that specifically inhibited hyperactive Cdk5/p25 and rescued cortical cells in vitro from abnormal AD-like phenotypes. It did this without affecting the function of the normal Cdk5/p35 and toxicity. In addition, the Intraperitoneal injection (IP) of TFP5 ameliorated ALS and PD phenotypes in model mice. This talk will focus on the role of TFP5 peptide as a therapeutic reagent to prevent AD, ALS and PD phenotypes in model mice
Keynote Forum
David Truswell
somefreshthinking ltd. UK
Keynote: The impact of dementia on migrant communities: A complex challenge in a globalised world
Time : 10:50-11:10

Biography:
David Truswell has worked in community based mental health services in the UK for over thirty years developing services for people with complex care needs and enduring mental health problems in a career spanning the UK voluntary sector, local authority services, and the NHS. From 2009 - 2011 he was the Dementia Implementation Lead for Commissioning Support for London. He is the Chair of the Dementia Alliance for Culture and Ethnicity, a grassroots alliance of dementia organisations. He recently left the NHS to set up somefreshthinking (www.somefreshthinking.com) a health sector change management consultancy. He is also an independent writer on dementia support and services for Black, Asian and minority ethnic communities
Abstract:
Statement of the Problem: While there is now worldwide recognition of the challenges that dementia brings to national health economies, less well understood is the impact of dementia as a health issue in migration. As migrant communities settle both lifetime health risks and the age structure of the population may place some migrant communities at greater risk of developing dementias such as Alzheimer’s disease and vascular dementia than the mainstream population. Delayed presentation for diagnosis, fear of discrimination and cultural stigma all increases the likelihood that individual cases will be complex in nature and that overall numbers will increase in those metropolitan areas across the globe that are often called ‘gateway cities’ for international migration.
Methodology & Theoretical Orientation: Both nationally and internationally the study of health issues for migrant populations has yet to single out dementia as a focus for policy development and research. The number of people involved and complexity of the impact becomes apparent when considering the situation in metropolitan areas with long settled and diverse migrant communities where a significant proportion of people in the migrant communities concerned are over 65 and there are identified population specific health issues that increase the likelihood of dementia.
Conclusion & Significance: This is a high cost per case population likely to present late to services with complex care needs that challenge available resources. Along with polypharmacy risks there is a lack of understanding of any population specific pharmacological risks. The increasing scale of international migration and historical pattern of migration will lead to an increase in demand for dementia services from migrant communities not only as a result of generally increases in longevity but also due to differential health risk and age profile structure within migrant communities.. Health service planners are ill prepared for this
Keynote Forum
Gjumrakch Aliev
GALLY International Biomedical Research Consulting LLC., USA
Keynote: Mitochondrial Lesions as an Earlier hallmark for Liver and Brain Neuronal Damage During the Chronic Ethanol Self Administration in Cynomolgus Monkeys: New Challenge for Treatment Strategy
Time : 09:55-10:20

Biography:
Dr. Gjumrakch Aliev, MD, PhD, President “GALLY” International Biomedical Research Institute Inc., San Antonio, Texas, USA. He also hold appointment with the University of Atlanta, Atlanta, Georgia, USA as a Professor of Cardiovascular, Neuropathology, Gerontology, Health Science and Healthcare Administration, and Leading Scientist in the Institute of Physiologically Active Compounds, Russian Academy of Sciences. He received his MD in 1982, from the Baku Medical University (former USSR) with cum laude. Then he accomplished his PhD in Cardiovascular Diseases from the prestigious Russian Academy of the Medical Sciences, Moscow, Russia in 1988 with cum laude. He received postdoctoral training with Professor G. Burnstock in the University College of the London. He authored and coauthored more than 500 publications in the fields of neurodegenerative diseases research (Alzheimer disease), as well as cardio- and cerebrovascular disease, cancer, and electron microscopy. He is an outstanding teacher, scholar, and a renowned scientist in the area of cellular molecular physiology, and cardiovascular, and neurodegeneration-mediated pathologies and drug development including Alzheimer disease (AD). He is nationally and internationally reputed in his area. Dr. Aliev’s accomplishments in the area of biochemistry and cellular biology have tremendous implications for drug design towards CNS Neurological Disorders, AD, cancer, and cerebrovascular and neurodegeneration related pathologies. He is world-renowned expert in electron microscopy. His work has been published in numerous prestigious journals such as Nature Clinical Cardiology, J. Neuroscience, Circulation Research, New England journal of Medicine, Blood, J. Cellular and Molecular Medicine, Atherosclerosis, CNS Neurological Disorders & Drug Targets, International J. Biochemistry and Cell Biology, Scientific Reports, and many others which reflect his leading role in his research areas. He is currently the Editor in Chiefs for “Central Nervous System Agents in Medicinal Chemistry”, “Applied Cell Biology”, “World Journal of Neuroscience”, “Open Journal of Psychiatry” and “Journal of Aging Science”, Cardiovascular & Hematological Agents in Medicinal Chemistry as well as which by itself shows the voluminous and outstanding work he has accomplished in the area of cellular and molecular biology as well as aged associated clinical sciences. He is one of most cited authors in his fields with high impact factors
Abstract:
Alcoholism is the third leading cause of preventable death in the United States and caused several neurological diseases. Aside from promoting cardiomyopathies, chronic alcohol consumption is associated with an increased risk of dementia, the development of liver or pancreas failure, and cancers of the oral cavity and pharynx. Although a J-shaped curve for all cause mortality has been identified for average alcohol consumption, irregular heavy drinking also carries significantly greater risks for cardiovascular and neurological disease. Alcohol induced cardiovascular and neurological diseases has a complex multigenic etiology. Significant variations in the response to chronic alcohol consumption may be related to unique genotypes that modify the metabolic response to ethanol. Future studies to further characterize the role of different genotypes will help identify those genotypes are more susceptible to chronic alcohol consumption.
Mitochondria are important for providing cellular energy ATP through the oxidative phosphorylation pathway. They are also critical in regulating many cellular functions including the fatty acid oxidation, the metabolism of glutamate and urea, the antioxidant defense, and the apoptosis pathway. Mitochondria are an important source of reactive oxygen species (ROS) leaked from the electron transport chain while they are susceptible to oxidative damage, leading to mitochondrial dysfunction and tissue injury. Several studies suggested that alcoholism causes impaired mitochondrial function leads to many types of neurodegenerative diseases.
Alcoholism may result in severe neurological deficits and cognitive impairments. Neuropathy and neurocognitive deficits are common among chronic alcohol users, which are believed to be associated with mitochondrial dysfunction in the brain especially cerebellum, cortex as well as liver. The specific type of brain mitochondrial ultrastructural lesions that are adversely affected by alcohol abuse has not been studied. Increasing evidence indicates that chronic alcoholism appears to be linked to oxidative damage and aging. However, the precise connection between chronic alcoholism and oxidative damage is unclear and is under investigation. Our recent gene expression analysis revealed that genes related to oxidative phosphorylation and longevity were down-regulated in the ethanol-fed monkeys, suggesting that alcohol may accelerate aging in monkeys by damaging their mitochondria
- Track 1: Neuroimmunology and Neuroinflammation | Track 6: Neurochemical Transmission | Alzheimer’s Disease and Dementia | Track 11: Parkinson’s Disease
Location: Hilton Garden Inn Milan North Via Lucio Giunio Columella, 36, 20128 Milano, Italy
Session Introduction
Christina Francisca Vogelaar
Johannes Gutenberg University Mainz, Germany
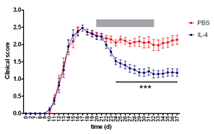
Title: Fast direct neuronal signaling via the IL-4 receptor reverses disease severity in progressive neuroinflammation

Biography:
Christina Francisca Vogelaar has her expertise in nerve regeneration. Her research focusses on the molecular mechanisms of peripheral and central axon regeneration and the treatment of traumatic and neuroinflammatory axon injury. She worked on sciatic nerve regeneration, on axonal RNA localization, and on spinal cord injury. In Mainz (Germany), her group works on ribosome transport and transfer in axons. She recently joined the lab of Frauke Zipp (Mainz, Germany) to investigate axon repair in neuroinflammation.
Abstract:
Marina Farinelli
Clinical Psychology Service “CASA DI CURA VILLA BELLOMBRA†rehabilitation hospital - Consorzio Colibrì - Bologna, Italy
Title: The Impact of Brain Lesions On Basic Emotions And Emotion Regulation In Stroke Survivors

Biography:
Marina Farinelli is MD, specialist in clinical psychology, psychotherapist and psychosomatic specialist (ICPM). Her main fields of interest for research and clinical practice are the psychosomatic approach and neuroscience. She taught at Bologna and Chieti Universities (Italy).
Working with neurological patients at Villa Bellombra Rehabilitation Hospital (Bologna) since 1996, she established and currently coordinates a Clinical Psychology Service to support patients and their caregivers, integrate the multi-professional team and carry out researches. Since 2009 she has been coordinating the research project devoted to stroke patients entitled “The impact of brain lesion on internalization/externalization processes: a neuropsychodynamic study” carried out in collaboration with the Neuroradiological Unit (IRCCS) of Bellaria Hospital of Bologna, the Dept. of Psychology of Bologna University and the Institute of Mental Health University of Ottawa. The clinical and research experiences and findings have been presented at numerous national and international congresses and published on scientific journals.
Abstract:
Statement of the problem: Panksepp (1998) first proposed an “affective neuroscience” approach to explore foundations of human and animal emotions in connection with specific subcortical brain systems which underlie the self (core Self) and the self-referential processing. In particular, four basic emotional systems (SEEKING, ANGER, FEAR and LUST) were identified having evolutionally deep reptilian roots and three (CARING, SADNESS and PLAY) reflect more uniquely mammalian adaptations. The Affective Neuroscience Personality Scales (ANPS) have been developed to evaluate these emotional, personality tendencies in humans. The alterations of basic emotions after brain injury according to Panksepp’s theory remain understudied. The aim of this presentation is to illustrate the outcomes of recent researches that studied basic emotions and emotion regulation in stroke survivors and their implication for pharmacological treatments and rehabilitation in clinical practice. Methodology: A sample group of stroke patients and a control group of orthopedic patients both hospitalized and in post-acute phase were evaluated by two self-report questionnaires: ANPS and HADS (Hospital Anxiety and Depression Scales) taking into account the location of brain damage. Findings: Stroke patients showed a statistically significant lower scores in ANPS-SEEKING and higher scores in depression by HADS in comparison with the control group. Moreover, further alterations in basic emotion systems in comparison to the control group were found, highlighting a specific pattern of emotion regulation. The involvement of anterior subcortical-cortical midline-structures was evident. Conclusions and significance: The specific changes and alterations of basic emotions and dispositions towards the external environment in relation to peculiar brain damage in stroke entail tailored stimulations and modulations of physiotherapeutic and pharmacological treatments during the rehabilitation process. Furthermore a tailored psychotherapeutic intervention often needs to support patients and their caregivers; the involvement of caregivers is necessary due to their role in emotion regulation in daily life.

Biography:
Varghese received his PhD from the Department of Medicinal Chemistry, University of Minnesota in 1985. He a postdoctoral fellowship in Professor Josef Fried’s lab in the Department of Chemistry, University of Chicago and a second postdoctoral fellowship in Professor Carl Djerassi’s lab in the Department of Chemistry at Stanford University. He worked with Athena Neurosciences/Elan Pharmaceuticals as a senior member of their Discovery team for 18 years. He then joined the Buck Institute for Research on Aging where he was Director of Alzheimer’s Drug Discovery Network. He started the Drug Discovery Lab at UCLA in 2015.
Abstract:
Apolipoprotein ε4 (ApoE4) is a major genetic risk factor for sporadic, late-onset Alzheimer’s disease (AD). One protein target that is affected in the presence of ApoE4 is the major longevity determinant and NAD-dependent deacetylase sirtuin-1 (SirT1), which we showed to be decreased in the presence of ApoE4 (Theendakara, PNAS 2013). Recent reports also show that SirT1 levels are shown to be decreased in serum of AD patients (Kumar, PLoS One 2013). Through screening we have identified a brain-penetrant small molecule, A03, that increased the neuroprotective SirT1 protein levels in ApoE4-transfected cells, and we have recently tested its efficacy in vivo in a ApoE4 mouse model for AD. The preliminary results show that A03 treatment can increase in SirT1 levels in the mouse brain thus providing initial proof-of-concept for developing this drug candidate as a ApoE4-targeted therapeutic for AD. We are in the process of designing and synthesizing analogs of A03, and studying their effects on both the neuroprotective SirT1 and neurotoxic protein sirtuin-2 (SirT2) in ApoE4-transfected cells. In addition, we have also initiated high throughput screening (HTS) of the UCLA compound library to identify new ‘hits’ that increase SirT1 in the presence of ApoE4. Our data thus reveal a novel mechanism for developing targeted therapeutics for this major known risk factor for AD. A03 is a promising lead candidate that increases brain SirT1 levels and could be developed as an ApoE4-targeted therapeutic for AD. Synthesis, testing, and screening of new ‘hit-analogs’ could yield additional candidates for development as potential therapeutics for Mild Cognitive Impairment (MCI) and/or AD. The research is supported by funding from NIH (R01AG051386) and the UCLA Easton Center for AD Research.
Alireza Rezayi
Shahid Beheshti University of Medical Sciences, Iran
Title: Intravenous Immunoglobulins for children Refractory Epilepsy

Biography:
Rezayi A.R has expertise in child neurology and he is working on rare disease such as neurometabolic and referactory epilepsy in thirthiary center of child neurology center(mofid children hospital and loghman hakim hospital) in Tehran, Iran.He is publicated several article in field of child neurology and he is faculty member of shahid beheshti university of medical sciences and interested in education of child neurology fellow and pediatric residents and medical students.He was certificated in Iranian child neurology national board in septamber2011 and he is membership in several international child neurology society such as ICNA(international child neurology association), AOCNA (asian and oceanian child neurology association),EPSN(European Paediatric Neurology Society) and Iranian child neurology association.
Abstract:
Statement of the Problem :This study was conducted to investigate the efficacy of intravenous immunoglobulin for drug-resistant seizures in children reffered to pediatric neurology clinic .
Methodology & Theoretical Orientation:A retrospective review of all children in loghman hakim hospital neurology clinic from 2012 to 2015, inclusive, with intractable epilepsy who were treated with intravenous immunoglobulin for a minimum of 3 cycles was performed. Data collected included seizure frequency, seizure and epilepsy syndrome type, propable etiology for the seizures, and. Response to intravenous immunoglobulin was defined as “positive” if either seizure freedom or ≥50% reduction of seizures was achieved.
Findings:fourteen children (8 m -12 years old) were included in the study. Treatment with intravenous immunoglobulin, the following outcomes were noted: Three were seizure-free, four had 90% reduction, two had 50% reduction,three had 30 % reduction and two had any responsivness. A total of 9 (64%) patients had a positive clinical response to IVIG treatment from baseline. Five patients (36%) were not responsive. No relationship of responsiveness to intravenous immunoglobulin with regard to age, gender, or epilepsy syndrome was detected.
Conclusion & Significance:Our study and others suggest that intravenous immunoglobulin can used in the treatment of children with drug-resistant epilepsies with potentially high efficacy and low side effect. This treatment was able to reduce multiple seizure types in a variety of epilepsy etiologies, including those of unknown cause or even inborn error of metabolism.
Eunji Cheong
Yonsei University, South Korea
Title: Thalamocortical circuit in sleep control: the thalamic mGluR1-PLCï¢4 pathway is critical in sleep architecture

Biography:
Eunji Cheong and her research group have expertise in studying electrophysiological properties of neurons from ion channel level to brain circuit level. Her lab has studied the thalamocortical circuit in controlling the vigilance state of brain such as sleep state control and pathological impairment of consciousness in mice model for many years. She also worked on studying the ion channels and synaptic transmission which control the excitability of thalamocortcial neurons. She has published over 30 research papers in prestigious journals during the last 5 years
Abstract:
The transition from wakefulness to a nonrapid eye movement (NREM) sleep state at the onset of sleep involves a transition from low-voltage, high-frequency irregular electroencephalography (EEG) waveforms to large-amplitude, low-frequency EEG waveforms accompanying synchronized oscillatory activity in the thalamocortical circuit. The thalamocortical circuit consists of reciprocal connections between the thalamus and cortex. The cortex sends strong excitatory feedback to the thalamus, however the function of which is unclear. Here we investigated the role of the corticothalamic inputs onto thalamcortical (TC) neurons via metabotropic glutamate receptor 1 (mGluR1) pathway in sleep control. The mGluR1 in TC neurons is linked to phospholipase C b4 (PLCb4) pathway. In PLCβ4-/- mice, the transition from wakefulness to the NREM sleep state was stimulated, and the NREM sleep state was stabilized, which resulted in increased NREM sleep. The power density of delta (δ) waves increased in parallel with the increased NREM sleep. These sleep phenotypes in PLCβ4-/- were consistent in TC-restricted PLCβ4 knockdown mice. Moreover, in vitro intrathalamic oscillations were greatly enhanced in the PLCβ4-/- slices. The results of our study showed that top-down control of thalamocortical circuit was critical in controlling sleep architecture.
Mariya Hristova
University College London, UK
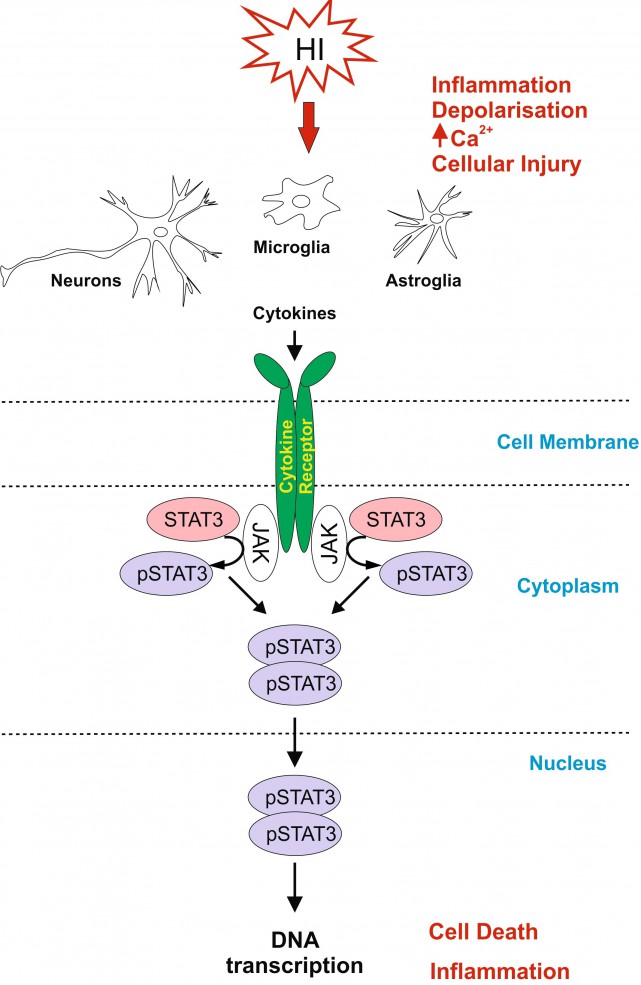
Title: Inhibition of Signal Transducer and Activator of Transcription 3 (STAT3) reduces neonatal hypoxic-ischemic brain damage

Biography:
Dr Mariya Hristova is Senior Research Associate and leads the Perinatal Brain Repair Group at the Institute for Women’s Health, University College London. She has very strong expertise in neuroimmunology and in the hypoxia-ischaemia model, therapeutic hypothermia and brain analysis investigating the role of post-translational modifications, transcription factors (STAT3), cytokines (TNFa) and pH changes following neonatal hypoxia-ischaemia. She has been an essential part of the team studying the combination of xenon and therapeutic hypothermia, and melatonin and therapeutic hypothermia in a neonatal piglet model of transient birth asphyxia.
Abstract:
Neonatal hypoxic-ischaemic encephalopathy (HIE) is a leading cause of neonatal mortality and morbidity, affecting 1-3 per 1000 live-births in developed countries with rates about 5-10 times higher in low-income setting. About 40% of the affected children die in the neonatal period and further 30% develop life-long disabilities such as cerebral palsy, epilepsy and mental retardation. Therapeutic hypothermia is the only clinically approved care for moderate to severe neonatal hypoxic-ischaemic (HI) brain injury, however it reduces death and disability only by 11% with about 40% of the treated infants still developing neurological incapacities. For hypothermia to be effective, 7-8 infants need to be treated for one to benefit from the treatment. Therefore it is necessary to develop simple, safe and effective supplementary therapies to add to the current therapeutic strategy in infants with HIE.
Signal Transducer and Activator of Transcription 3 (STAT3) is strongly up-regulated by HI in the immature brain. To investigate the association of STAT3 up-regulation with HI-brain damage, and whether phosphorylated STAT3 originating from different cell types has a different role in promoting HI-damage, we subjected postnatal day seven mice to unilateral carotid artery ligation followed by 60min hypoxia. Neuron-specific STAT3-deletion reduced microglial and astroglial activation, cell death and tissue loss in all brain regions. Astroglial STAT3-deletion also reduced microglial activation, cell death and tissue loss, although not as much as neuronal deletion. Systemic STAT3-inhibition with JAK2-inhibitor WP1066 only moderately reduced microglial and astroglial activation, but in a pattern similar to the one observed with the cell-specific deletions. Our results suggest that STAT3 is an important factor in neonatal HI-brain damage and its removal in neurons or astrocytes, and, to some extent, systemic inhibition reduces inflammation and tissue loss. Overall, the protective effects of STAT3 inhibition make it a potential target for a therapeutic strategy in neonatal HI.
Joel B Schachter
Merck Research Laboratories, USA
Title: Can Tau Pathobiology be Pheno-copied in a Cellular Expression Model?

Biography:
Joel Schachter is currently a Principal Scientist in the Movement Disorders and Translation Group at Merck. Dr Schachter joined Merck in 2009 as Director of Neurology and has held several positions there including head of External Discovery and Preclinical Sciences for Neuroscience (XDPS) and Franchise Collaboration Lead for Neuroscience. In his role within XDPS, he was involved in building effective collaborations with external partners and in execution of preclinical drug discovery programs in the areas of Alzheimer's Disease, Parkinson's Disease, Schizophrenia, and pain/migraine. As Franchise Collaboration Lead, he developed collaborative basic research efforts with academic partners. Prior to his work at Merck, Dr Schachter spent 12 years at Pfizer as a member of the Molecular Sciences Department and the Neurology group where he led project teams for several Alzheimer’s Disease-related drug discovery programs including GSK3, Gamma modulators, and Tau New Targets
Abstract:
Tau hyperphosphorylation and formation of insoluble tau deposits are principal aspects of the neurofibrillary pathology associated with Alzheimer’s disease (AD) and other neurodegenerative Tauopathies. Despite a histology-based focus on insoluble filamentous tau pathology, small soluble tau oligomers have recently been implicated in the promotion of neurodegenerative activities and are now widely viewed as central participants in disease-related neurodegeneration. The factors that initiate the aberrant post-translational processing of tau and the generation of toxic tau oligomers are not well defined, but hyperphosphorylation has been implicated to play a critical role in tau aggregation. We have generated a cellular model in which full length human tau is expressed as a dimer-like structure that we refer to as “tandem repeat tau” (TRT). Cellular expression of TRT results in rapid hyperphosphorylation of the protein at disease-relevant epitopes. The rapid hyperphosphorylation is followed by a slower formation of high molecular weight tau oligomers that are stable to detergent extraction and gel filtration. TRT displays proteolytic processing with multiple cleavage products that are not observed for the monomeric version of tau. Cells expressing TRT show increased propensity for caspase activation and an activation of the unfolded protein response, suggesting that TRT expression initiates a cascade of pathological cellular responses that compromise cellular viability. Given multiple observations of similar post-translational processing of TRT in this model, compared to tau pathology in brains of patients with AD and other neurodegenerative Tauopathies, we suggest that this model may be useful for delineating cell biology associated with Tauopathy, as well as providing a model system for testing potential therapeutic agents.
Aruna Sharma
Uppsala University, Sweden
Title: Nanodelivery of cerebrolysin induces neuroprotection in Parkinson’s disease

Biography:
Aruna Sharma, MD is currently Secretary of Research at Uppsala University Hospital, Uppsala University, Sweden. She obtained her Bachelor of Science in 1971 and trained in Indian Medicine up to 1977 and engaged in medical research from 1978 to 1986 in India on hyperthermia induced brain dysfunction in the lab of Hari Sharma and Prasanta Kumar Dey under University Grants Commission and Indian Council of Medical Research Programs. She is a qualified experimental Neurpathologist and received her training at Karl Marx University Leipzig, Institute of Neurobiology (1987-1988); Semmelweis University Medical School, Department of Human Morphology and Developmental Biology, Budapest, Hungary (1988-1989), Free University Berlin, Germany (1989-1991) and Neuropathology Institute Uppsala (1992-1995). Dr Sharma is member of various Distinguished American Organizations and elected to receive the prestigious award “Women of the Years Representing Sweden Award 2009” for her outstanding contributions towards society by American Biographical Research Institute, USA; and “Best Professional Business Women Award 2010” For Setting Standard to Motivate, Excel and Inspire Others, Raleigh, North Carolina, USA. She has published over 50 original research papers in Reputed Neuroscience Journals and is currently Acquisition Editor of American Journal of Neuroproetction and Neuroregenartion.
Abstract:
Parkinson’s disease (PD) affects over 80 thousand Americans every year for which no suitable therapy is available till date. PD induces severe disability in victims and so far no suitable strategies have been developed resulting an urgent need to explore novel therapeutic strategies to treat PD for the benefit of the mankind. Recently, nanodrug delivery of therapeutic compounds has been shown to induce superior neuroprotective effects than the parent compounds in central nervous system (CNS) diseases.
There are evidences that increased oxidative stress and neurotoxic elements in the CSF and in the brain appears to be responsible for PD pathology and decline in cognitive and motor functions. Recent research suggests that increased alpha-synuclein (ï¡-synuclein, ASNC) in the CSF and in several brain areas together with oxidative stress correlates well with the brain pathology and cognitive decline in human cases of PD. Thus, a possibility exists that drugs that are capable to reduce the levels of oxidants and/or ASNC could be useful for novel therapeutic tools in PD.
Previous reports from our laboratory showed that intraperitoneal injections of 1-metyl-4-fenyl-1,2,3,6-tetrahydropyridin (MPTP, 20 mg/kg) daily within 2-h intervals for 5 days in mice induce PD like symptoms on the 8th day. This model is well-characterized biochemically, histologically and functionally for PD like symptoms. Thus, marked decrease in the number of tyrosine hydroxylase (TH) positive cells in the Substantia Nigra Pars Compacta (SNpc) and striatum (STr) as well as decrease in dopamine (DA) and its metabolites 3,4-Dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) with marked behavioral dysfunctions e.g., Rota-Rod performances, walking on an inclined mesh grid and gait deficits was seen on the 8th day.
Cerebrolysin (CBL) is a well-balanced composition of several neurotrophic factors and active peptide fragments. Thus, this multimodal drug may have an added value on therapeutic strategies in PD. In present investigations we examined timed release of CBL using titanate nanospheres (TiNS) in treating PD in our mouse model. In this investigation, timed release of CBL using titanate nanospheres (TiNS) treatment results in significant neuroprotection and behavioral improvements. Thus, it would be interesting to examine whether this model of PD is also associated with increased ASNC and free radical nitric oxide in the CSF and brain areas of mice and cerebrolysin treatment could modulate these elements in our model.
ASNC was measures using commercial ELISA kit in the CSF and in brain whereas neuronal nitric oxide synthase (nNOS) was examined using immunohistochemistry on paraffin sections. Our results showed a significant increase in ASNC by 2- to 4-fold in the CSF and in various brain areas from normal control group (control: SNpc 1.78±0.08 ng/mg, STr 6.34±0.21 ng/mg, frontal cortex 8.24±0.32 ng/mg; CSF 1.21±0.07 pg/ml). In these brain areas nNOS expression was also increased by 4- to 8-fold as compared to control group. Nanodelivery of cerebrolysin (3 ml/kg, i.v. 2 days after MPTP for 5 days) significantly reduced ASNC levels in the CSF and in all the brain areas examined. In the treated PD mice downregulation of nNOS was also seen in the above brain regions. These results are the first to show that nanodelivery of cerebrolysin induces neuroprotection in PD by reducing ASNC and nNOS expression. However, further research is needed to explore TiNS in clinical situations, a feature that requires additional investigations.
Ying-Chieh Tsai
National Yang-Ming University, Taiwan
Title: Psychobiotic PS128 rescued motor deficits in MPTP-induced mice model

Biography:
Abstract:
Parkinson’s disease (PD) is a common neurodegeneration disease caused by dopaminergic neuron degeneration in brain. The dopamine signaling collapsed and resulted in motor deficits like shaking, difficulty with walking and gait in PD patients. Recent studies have revealed that gut microbiota influence neurodevelopment, modulate behavior, and contribute to neurological disorders through microbiome-gut-brain axis (MGBA). Certain probiotics strain, or “psychobiotics”, even showed unique psychotropic effects in many animal studies and clinical trials. Lately, we found a special psychobiotic Lactobacillus plantarum PS128 which improved dopamine transmission in brain specific regions and modulated behaviors in different mice models, raising the possibility that that PS128 might show beneficial effect on host’s CNS dopamine system through MGBA. In this study, we used 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to establish a PD-like mice model to investigate whether PS128 also show neuroprotective effect on host’s CNS dopamine system. PS128 was oral administered to mice for 4 weeks before 5-day MPTP injection. We found PS128 significantly improved the pole test, narrow beam test, and rotarod test performance, indicating that it rescued MPTP-induced motor deficits. Further brain tissue analyses showed that PS128 prevented MPTP-induced dopaminergic neuron loss in substantia nigra and rescued dopamine & noradrenaline total level in striatum. In conclusion, PS128 could prevent MPTP-induced motor deficits, dopaminergic neuron loss, and neurotransmitter signaling collapse. PS128 might show neuroprotective potential on host’s CNS dopamine system leading to clinical application for treating and preventing PD or other dopamine-related neuropsychiatric disorders.
Image
Psychobiotic PS128: start with mental health, step forward to brain health
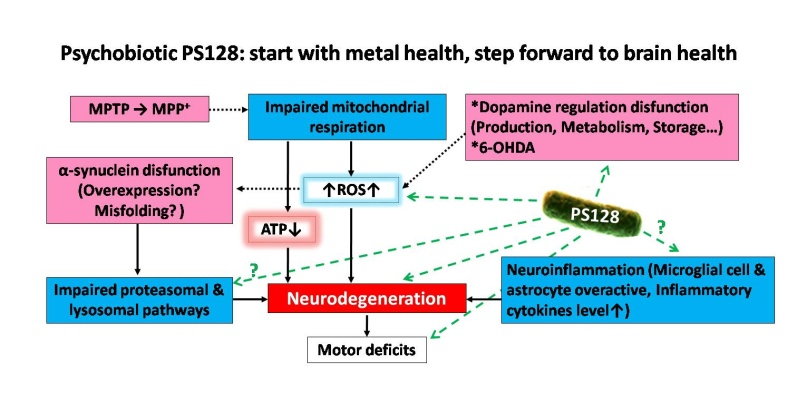
Figure 1: The proposed pathways of Parkinson’s disease (PD) & PD-like animal models and the possible mechanisms of PS128 neuroprotective effects on host’s CNS dopamine system.
Elizabeth K Barber
Barber Innovations LLC, USA
Title: Therapeutic Strategies for the Parkinson's Patient with Alzheimer's Changes: The Benefits of Art, Structure and Nutrition

Biography:
Elizabeth Barber has a long interest in integrative medicine, extensive experience as a Parkinson's Disease (PD) care manager as well as graduate work in signal transduction. She completed her BA with Honors, receiving the Robie Medal for outstanding graduate then Rotary Ambassadorial Scholar. At Harvard, she completed a master's in Biology, co-authoring several publications. Her doctorate at Oxford University on CD33 and CD34 in Biochemistry included neuromuscular disease. She became a trial lawyer/advocate at in London which she then applied to organize the many care, administrative and advocacy issues of PD from 2003 to 2015. She was invited to join the American Academy of Neurology in 2005 which selected her as a Palatucci Leadership Forum Advocate. She combined her knowledge of Art, interior design and science to develop an integrative care plan, serendipitously discovering a PD treatment, starting Barber Innovations LLC to develop her research while managing patient care.
Abstract:
Statement of the Problem: Parkinson's Disease (PD) has traditionally been treated mainly with drugs upon presentation of a tremor, stiffness or weakness by the patient which usually progresses to include "Alzheimer's Changes" or PD dementia when the patient is often transferred to a nursing facility. Epigenetics and nutragenomics show that we can improve our health through diet and lifestyle changes, as certain pesticides are known to cause PD.1 Staying in the home is generally less expensive than a nursing home with better health outcomes. Methodology and Theoretical Orientation: This case study presents the long-term therapeutic benefits of a preventive strategy creating a positive and safe neurological environment. A combination of therapeutic art,2 design, structured meals, medication, supplements, exercise, reducing environmental and food toxins was integrated to develop a workable strategy for providers and families to manage the disease. Following a fall and hospitalization, a stepwise program was developed and implemented in the home using the patient's preferences and research to allow for a smooth transition from rehabilitation into a neurologically therapeutic environment integrating art and healing interior design.3 The environment was made safer, a system of medication and meal administration highlighting neurologic and intestinal health was implemented and a caregiver instruction system was established. Certain nutritional supplements4-5 and regular exercise were integral treatment arms. A team approach to care was emphasized, including family members and providers. Findings: The combination of therapeutic approaches and strategies was found to be particularly helpful and significantly increased the patient's lifespan as well as lowering the home stress level. The therapeutic strategies were put into a teaching tool, so that new caregivers and family members could be taught the program and prepare for disease progression. Conclusion & Significance: The checklist of strategies used at different stages of the disease will aid providers, patients and their families in coping with and ameliorating PD difficulties.
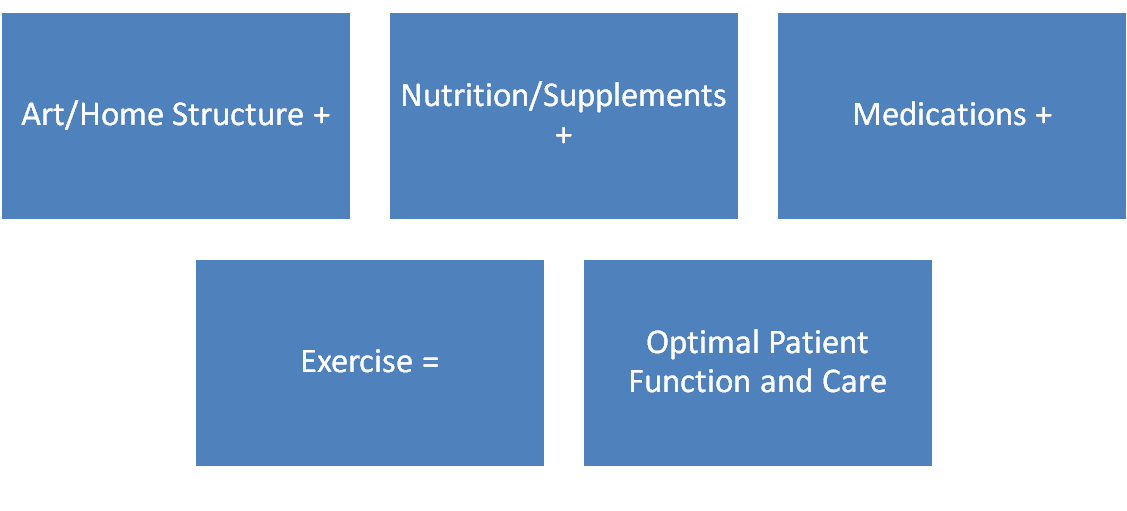
Figure 1. Integrated Aspects of Parkinson's Disease Care. An effective care plan includes Nature Art, a Structured Home Environment, Targeted Nutrition and Supplements, Scheduled Medication and Targeted exercise for optimal patient function and care.
Michel CYR
University of Trois-Rivieres, Canada
Title: Oral treatment with sphingosine-1-phosphate receptors modulator prevents Parkinson symptoms concomitantly to reduced brain inflammation in MPTP mouse models

Biography:
Michel CYR is a PhD holder in Pharmacy from Laval University in Canada and a Post-Doctoral Trainee in Cellular Biology at Duke University in the USA. He is currently a Full Professor at the Medical Biology Department at the University of Trois-Rivieres. As the Director of the Canada Research Chair in Molecular Neuropharmacology, he is leading the effort to understand the molecules in the brain that are responsible for learning and executing motor actions. He is also investigating the molecular bases for movement disorders such as Parkinson disease and makes it possible to better target and develop new treatments.
Abstract:
Here we explore the efficacy of an oral treatment with fingolimod (FTY720), a selective sphingosine-1-phosphate receptors modulator, to prevent MPTP induced nigrostriatal loss and motor deficits in mice. In addition, potential molecular mechanisms have been assessed. Sphingosine-1-phosphate is a potent bioactive lipid mediator that acts as a natural ligand upon binding to five different receptors that are located in astrocytes, oligodendrocytes, microglial and neuronal cells. Modulation of these receptors has been shown to provide neuroprotection in multiple sclerosis and in mouse model of Alzheimer’s disease. Whether the selective sphingosine-1-phosphate receptors modulator FTY720 exhibits neuroprotection in Parkinson’s disease is unclear. Adult male mice were disseminated into four independent groups: vehicle (saline), FTY720, MPTP/vehicle and MPTP/FTY720. Chronic oral FTY720 (1 mg/kg) administrations began two days before the MPTP (30 mg/kg, i.p., 5 days) treatments. Motor behavioral tests and Western blot analyses on striatum tissues were assessed on these mice. We revealed diminutions of ~50% in the levels of tyrosine hydroxylase and dopamine transporter proteins in the striatum of MPTP mice. At the behavioral level, these mice exhibited motor deficits at the Pole and Beam tests. Interestingly, FTY720 oral treatment has the capacity to prevent these known detrimental signs associated to MPTP treatment. Further study has revealed that while striatal levels of phosphorylated extracellular signal-regulated kinases and S1PR subtype 1 were unaffected, tumor necrosis factor-alpha and glial fibrillary acidic protein levels were robustly increased in MPTP-treated mice, an outcome that was totally prevented by FTY720 treatments. Notably, FTY720 treatments was also able to prevent the reduction of brain-derived neurotrophic factor levels observed in the striatum of mice treated with MPTP. Our findings propose that oral FTY720 treatments prevent the damaging effects of MPTP on striatal dopamine terminals and motor behaviors. The mechanism of action may involve inhibition of inflammatory pathways and the modulation of brain-derived neurotrophic factor synthesis. This study is providing novel evidence for the clinical utility of targeting S1PR in Parkinson’s disease therapy.
Safa Najmi Tabrizi
Tabriz University of Medical Science, Iran
Title: Addiction and Withdrawal of Dopamine Agonists Therapy for Parkinson’s disease

Biography:
Dr. Safa Najmi Tabrizi, Born in 1972 in Tabriz, Iran; is an Assistant Professor of Neurology in Tabriz University of Medical Science in Iran. After Graduation in Neurology as a board certi,ied, he trained in Parkinson’s disease and Dementia in Istanbul medical University. Also, he was a research fellow in Washington University at Saint Louis and Saint Louis University in USA in 1ield of Dementia and Neurodegenerative disorders. He has had many lectures as an invited speaker in several international meetings and congresses. Safa Najmi has many articles and publications at different scienti4ic journals.
Abstract:
Hossein Ali Ebrahimi
Kerman University of Medical Sciences, Iran
Title: Multiple sclerosis and mines

Biography:
I am Hossein Ali Ebrahimi Meimand, Professor of neurology in neurology department of Kerman University of medical Sciences. I was born in Shahrbabak city of Kerman province of Iran at 15/3/1954. The my interests in neurology are epilepsy and multiple sclerosis, I have many articles in national and international indexed journals, more than 80 articles and near 100 abstracts in medical congresses, and I wrote a book about guideline of epilepsy management (Persian language). At know I am head of Neurology Research Center in Kerman University of Medical sciences, Kerman, Iran.
Abstract:
Multiple sclerosis (MS) is the most common inflammatory-demyelinating disease of the central nervous system.
Our knowledge about its pathogenesis is still incomplete and etiology remains unknown, early observations showed that the prevalence of MS is variable among geographic areas. One of the most geographic differences among areas of the world is mines existence. Also, available articles provide evidences of the effect of metals in MS pathogenesis. For example, iron, selenium, zinc, cooper.
Kerman province is a vast region with an area of 714,181 square kilometers located in southeastern Iran (latitude 30° north). Epidemiology of MS in different area of Kerman province are varied. Of course we observed an latitudes differences in different areas of Kerman province.
A cross-sectional study was conducted on MS patients in Kerman province in 2012. The details of the patients including age, sex, age at the onset of disorder and disease duration were collected from documents of MS centers of all cities. Diagnosis was confirmed according to the revised Mc-Donald criteria.
The climate varies in different parts of Kerman province. The rate of MS patients in Sirjan city with mines of iron is near 32, Rafsanjan city with some cooper mines is 27,and in Zarand city with a lot of coal mines is 27, these cities are dry and with an average temperature range of 16–20°C. The rate MS patients in Kahnuj city is near 2, Bam 17.5, and Jiroft, 14, these cities are warm and semi humid weather (average temperature of >20°C).
The mean prevalence of MS in mineral areas in Kerman province (Kahnoj, Sirjan, Zarand, Rafsanjan, Baft, Shahrbabak) was 23.01±10.78 and in non-mineral areas (Kerman, Bam, Jiroft) was 39.56±20.34 that show the higher prevalence of MS in non-mineral areas of Kerman province. A linear relationship between an increase in prevalence and low average temperature was observed. In the town of Shahrbabak which has cold weather, prevalence was low, which might be related to the presence a lot of copper mines in this area, because the ccooper is used in the synthesis of myelin.
Ola Ahmed Heikal
National Research Center, Egypt
Title: Rice bran, a Functional extract, new scientific evidences for potential health benefits in Alzheimer’s disease therapy

Biography:
Professor of analytical toxicology and head of department at The National Research Center (NRC) 2003, and professor in German university in Cairo (GUC) previously head of department of pharmacology & toxicology; faculty of pharmacy ( 2003-2013). Earned Ph.D from Japan (1993-1997). My Researches have been focused on the developing and application of analytical techniques in the field of toxicology, drug toxicokinetics , genotoxicity of nanoparticles and neurodegenerative diseases therapeutic approaches from natural origin . Lately published a number of articles in toxicological assessments of nutraceuticals. Involvement in national & international projects in cooperation with industry, to solve current problems or to innovatively develop market driven products form natural sources such as ; Combating Stunting in Qaliubia Egypt (CO PI, financed by Misr El Kheir Foundation). Rice bran Nutraceuticals and Rice Bran innovative formulation (Partner, Financed by the EU). Pharmaceutical Pectin from Orange Peels (Consultant, financed by Science and Technology Development Fund).
Abstract:
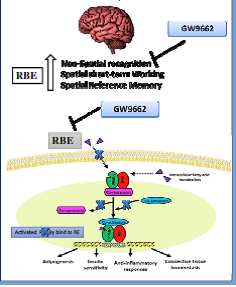
It is estimated that more than 500000 Tons of rice bran is produced in Egypt every year. Though its nutritional values and potential health benefits, it is used , due to its instability , as animal feed rather high value functional food and/ or high value added nutraceuticals. A number of papers were published showing, the stabilized rice bran extract, potential health benefits in some diseases like Alzheimer’s disease (Hagel et al., 2013 2015 a,b, c). The product is registered (Oryza), another one under registration at the Egyptian Ministry of Health (Riciplex) . A functional food against Alzheimer is currently developed in Germany, based on the Stabilized Egyptian rice bran supplied (Porridge Plus 6). In the present study the effects of RBE were examined in comparison to a well-known PPARγ agonist pioglitazone. RBE administration significantly improved the spatial working and reference memory in addition to non-spatial recognition memory in the LPS mouse model as shown by object recognition test, y-maze and water maze test. Pioglitazone improved memory, in the Y-maze and object recognition test with no effect in the water maze test. Interestingly, the effect of RBE on memory was abolished in the group injected with PPARγ-antagonist before RBE treatment, indicating the important role of PPARγ in the mechanism of action of RBE. Furthermore, the RBE -PPARγ DNA binding activity was measured in the brain extract samples of the mouse treated groups using transcription factor assay kit. Results showed a significant increase in PPARγ binding to PPRE with RBE treatment and this effect was reversed upon PPARγ antagonist injection before RBE treatment. These findings demonstrate that the involvement of RBE in the beneficial effects on cognitive performance is correlated with its action on PPARγ modulation, providing novel insight into its neuroprotective role in AD.
Koorosh Shahpasand
Royan Institute for Stem Cell Biology and Technology, Iran
Title: A Major Early Driver Of Tauopathy And Neurodegeneration That Is Blocked By Antibody

Biography:
Abstract:
Mona H Tawfik
Beni suef University, Egypt
Title: Cognitive Functions in Patients with Parkinson’s Disease: I. The Effect of Cerebral Microstructural Changes

Biography:
Mona Hussein Tawfik, has Master and MD degree in neurology from neurology department, Cairo University. She works as a neurology lecturer in Beni suef University. She has her expertise in evaluation and assessment of cognitive impairment. She has built her knowledge after years of experience in research in cognition. She has many publications about cognitive impairment in Parkinson's disease, Alzheimer's disease and normal aging.
Abstract:
Cognitive impairment in Parkinson’s disease (PD) was extensively studied in the medical literature. Correlating such cognitive impairment with the macro and microstructural changes in cerebral grey and white matter, has gained more attention in the last years. Aim: To explore the cognitive profile of patients with PD and to correlate the brain atrophic changes and the microstructural changes in cerebral grey and white matter with the cognitive pattern in Parkinson's disease. Subjects and methods: The study was conducted on 40 patients with PD and 20 controls. Selected PD patients were submitted to evaluation of cognitive function using PD-Cognitive Rating Scale (PD-CRS), and assessment of microstructural changes in substantia nigra (SN), caudate, putamen, globus pallidus (GP), thalamus, hippocampus and prefrontal white matter using diffusion tensor imaging (DTI). Results: The cognitive impairment in PD patients starts with executive dysfunction followed by impairment in attention, episodic memory, and visuospatial skills. Naming is the last cognitive domain to be affected in PD patients. The cognitive impairment in PD patients can be attributed to the microstructural changes (decreased Fractional anisotropy) in SN, caudate, putamen, GP, thalamus, hippocampus and prefrontal white matter. Conclusion: Cognitive impairment in PD is present even in the earlier stages of the disease and it can be correlated with the microstructural changes in SN, caudate, putamen, GP, thalamus, hippocampus and prefrontal white matter